The Honors Chemistry Writing and Balancing Equations Worksheet is a comprehensive resource designed to enhance students’ understanding of chemical reactions and equip them with the skills to write and balance chemical equations accurately. This worksheet provides a structured approach to mastering these fundamental concepts, fostering critical thinking and problem-solving abilities.
The worksheet encompasses various sections that cover the significance of balancing equations, step-by-step instructions for balancing, common errors to avoid, and an exploration of different types of chemical reactions. It also includes practice problems with varying difficulty levels to reinforce learning and assess understanding.
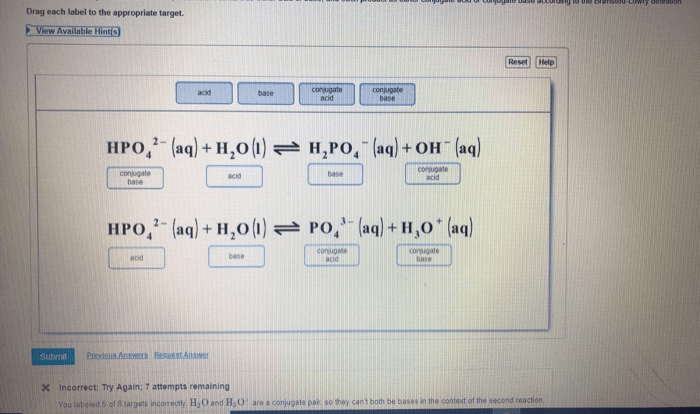
Writing Balanced Chemical Equations
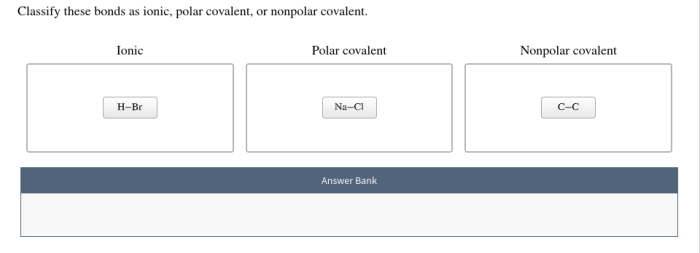
Chemical equations are symbolic representations of chemical reactions. They provide a concise way to represent the reactants, products, and stoichiometry of a reaction. Balancing chemical equations is essential for understanding the stoichiometry of reactions and for predicting the amounts of reactants and products involved.
To balance a chemical equation, the following steps can be followed:
- Write the unbalanced equation.
- Identify the atoms that are not balanced.
- Adjust the coefficients of the reactants and products to balance the atoms.
- Check the equation to make sure that all atoms are balanced.
Some common errors made when balancing chemical equations include:
- Changing the subscripts of the reactants or products.
- Adding or removing atoms from the equation.
- Not balancing all of the atoms in the equation.
Types of Chemical Reactions
Chemical reactions can be classified into different types based on the changes that occur during the reaction. Some common types of chemical reactions include:
- Combination reactions: Two or more substances combine to form a single product.
- Decomposition reactions: A single substance breaks down into two or more products.
- Single-replacement reactions: One element replaces another element in a compound.
- Double-replacement reactions: Two compounds exchange ions to form two new compounds.
The type of chemical reaction can be identified based on the reactants and products involved. For example, a combination reaction will have two or more reactants and a single product, while a decomposition reaction will have a single reactant and two or more products.
Honors Chemistry Writing
Honors chemistry writing requires students to demonstrate a high level of understanding of chemistry concepts. Students should be able to write clearly and concisely, and they should be able to use correct grammar and punctuation. In addition, students should be able to support their arguments with evidence from the scientific literature.
Here are some tips for improving writing skills in chemistry:
- Read scientific articles and books to learn how professional chemists write.
- Practice writing chemical equations and reaction mechanisms.
- Get feedback from your teacher or a tutor on your writing.
Here are some examples of well-written chemistry paragraphs:
The combustion of hydrocarbons is a complex process that involves the reaction of hydrocarbons with oxygen to produce carbon dioxide and water. The rate of combustion is affected by a number of factors, including the type of hydrocarbon, the temperature, and the presence of a catalyst. The combustion of hydrocarbons is an important process in many industrial applications, such as the production of energy and the manufacture of chemicals.
Worksheet Design
The worksheet should include a variety of practice problems on writing and balancing chemical equations. The problems should be designed to cover different levels of difficulty. The worksheet should also include answer keys or solutions to the problems.
Here are some ideas for practice problems:
- Write the balanced chemical equation for the combustion of methane.
- Balance the following chemical equation: 2Fe + 3Cl2 → 2FeCl3
- Identify the type of chemical reaction for the following equation: NaCl + AgNO3 → AgCl + NaNO3
Classroom Implementation
The worksheet can be used in the classroom in a variety of ways. It can be used as a homework assignment, a classwork assignment, or a review sheet. The worksheet can also be used to assess students’ understanding of writing and balancing chemical equations.
Here are some ideas for differentiating the worksheet for students with different needs:
- For students who are struggling, provide them with more scaffolding. For example, you could provide them with a partially balanced equation or a list of the reactants and products.
- For students who are ahead, challenge them with more difficult problems. For example, you could ask them to write the balanced chemical equation for a reaction that involves multiple steps.
Question Bank: Honors Chemistry Writing And Balancing Equations Worksheet
What are the benefits of using this worksheet?
The worksheet provides a structured and comprehensive approach to learning about writing and balancing chemical equations, enhancing understanding and problem-solving skills.
How can I access the worksheet?
The worksheet is typically provided by teachers or instructors as part of course materials.
What types of practice problems are included in the worksheet?
The worksheet includes a variety of practice problems with varying difficulty levels, covering different types of chemical reactions and scenarios.