Classify these bonds as ionic or covalent – In the realm of chemistry, understanding the nature of chemical bonds is paramount. This article delves into the fascinating world of ionic and covalent bonds, providing a comprehensive guide to their classification and characteristics.
Chemical bonds are the forces that hold atoms together to form molecules and compounds. Understanding the type of bond formed between atoms is crucial for comprehending the properties and behavior of chemical substances.
1. Explain the Concept of Ionic and Covalent Bonds
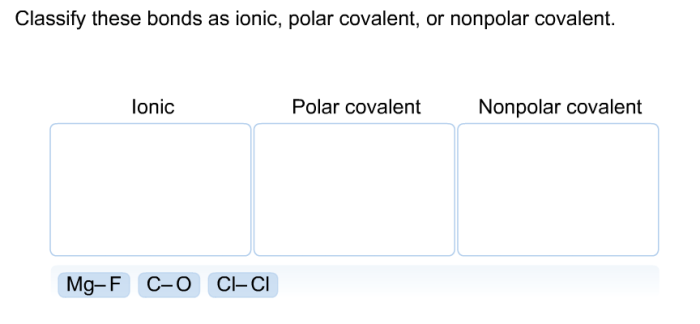
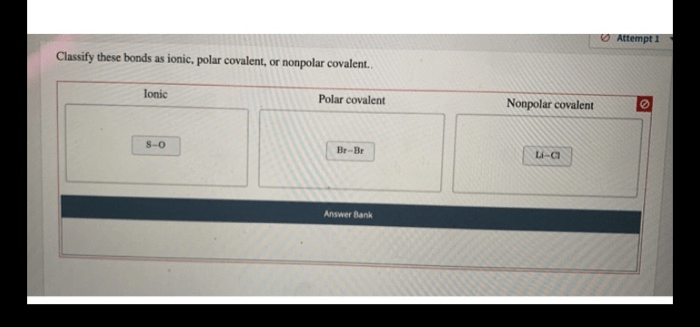
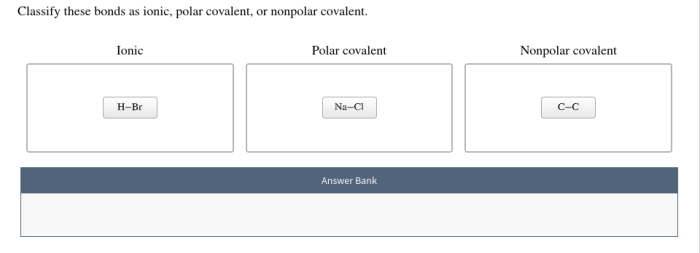
Ionic and covalent bonds are the two main types of chemical bonds that hold atoms together to form molecules and compounds. Understanding their differences is crucial for comprehending the behavior and properties of chemical substances.
Ionic bondsare formed between atoms with a large difference in electronegativity, a measure of an atom’s ability to attract electrons. In an ionic bond, one atom donates an electron to the other, creating positively and negatively charged ions. The electrostatic attraction between these oppositely charged ions holds the bond together.
Covalent bonds, on the other hand, are formed when atoms share electrons. These shared electrons are located in a region of space between the atoms, forming a covalent bond. Covalent bonds are typically formed between atoms with similar electronegativities.
Popular Questions: Classify These Bonds As Ionic Or Covalent
What is the key difference between ionic and covalent bonds?
Ionic bonds involve the complete transfer of electrons, resulting in the formation of ions, while covalent bonds involve the sharing of electrons between atoms.
How does electronegativity influence bond classification?
Electronegativity measures the attraction of an atom for electrons. A large difference in electronegativity between atoms leads to ionic bond formation, while a small difference results in covalent bond formation.